Animal research at Parkinson's UK
Animal research is currently essential in the search for better treatments and a cure for Parkinson's. But we are still committed to reducing this wherever possible.
Why do we use animals in research?
There is an urgent need for ongoing research in order to advance our understanding of Parkinson's, improve treatments and ultimately find a cure.
The use of animals is currently an essential tool in this research. In fact, it’s a legal requirement for any new medications.
But we are committed to the minimum possible use of animals and to ensuring the highest regulatory standards are maintained.
How have animals been used in Parkinson’s research?
Animal research has a long history of advancing the understanding of Parkinson's. In 1970, animal research was crucial in the discovery that dopamine was affected in people with Parkinson's, and the specific area of the brain where the dopamine producing cells were being lost. You can find out more about that research on the Globe and Mail website.
Animal research has also been essential in the development of deep brain stimulation, a type of surgery which can be used to treat Parkinson’s in some people. Find out more about deep brain stimulation.
What do we mean by animal research?
Much of our research can now be done using cells grown in a dish, computer modelling, clinical trials, or even using human tissue samples, such as donations to the Parkinson’s UK Brain Bank.
However, there are some things that can’t yet be tested without the use of an animal. This is known as in vivo research, which is research involving a living organism. This kind of research is invaluable when it comes to studying how some drugs might impact the body and the brain, or seeing how a condition like Parkinson’s develops over time.
In vivo research is also a required part of safety testing in the drug development process, according to current regulations in the UK. That means that almost every medical advance will have relied on animal research at some point during its development. We cannot deliver new treatments to the people who need them without the use of animals.
Learn more about research involving animals
In 2024, we were funding 55 cutting edge research projects across the UK. The number of active projects that involve animal research has varied over the last 5 years. At the moment, around 1 in every 11 of our active projects use animals in some way.
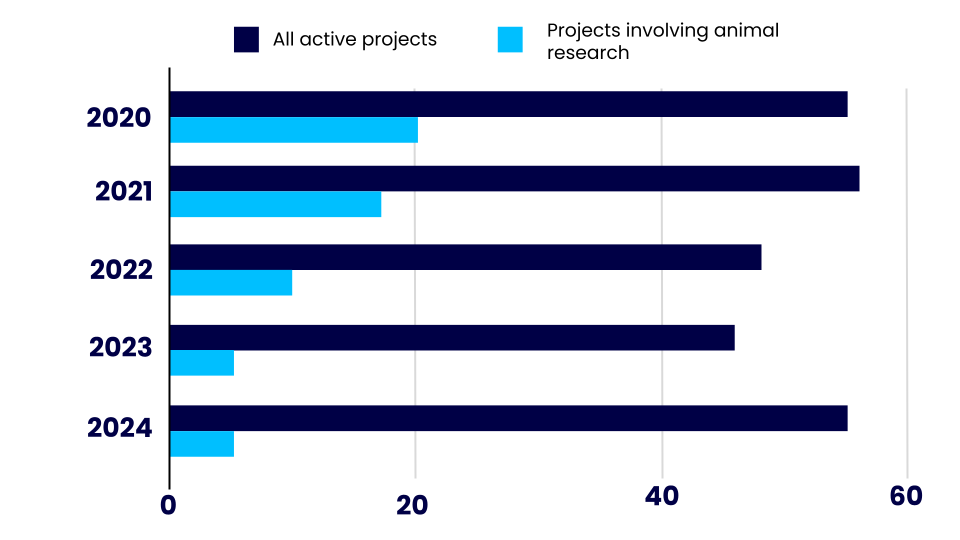
- In 2020 we had 55 active projects, 20 of which involved the use of animals.
- In 2021 we had 56 active projects, 17 of which involved the use of animals.
- In 2022 we had 48 active projects, 10 of which involved the use of animals.
- In 2023 we had 46 active projects, 5 of which involved the use of animals.
- In 2024 we had 55 active projects, 5 of which involved the use of animals.
Most of the research we fund through grants involves mice, as they offer a good way to examine specific proteins or genes to understand how potential drugs might work. Other commonly used species are rats, zebrafish, fruit flies, or small worms known as C. elegans.
Each year we fund new research projects. The number of projects varies each year. In 2024 we awarded funding to 16 new projects which started that year.
New funding applications are reviewed by our panel of scientific experts and lay grant reviewers who have a personal connection with Parkinson’s.
When animal research is involved, the panel will check that the researcher has made it clear that there is no way of answering their specific research question without using animals. And that the research aims reflect the priorities of people living with the condition.
In 2024, 1 new research grant was awarded to a project that plans to use animals in their research.
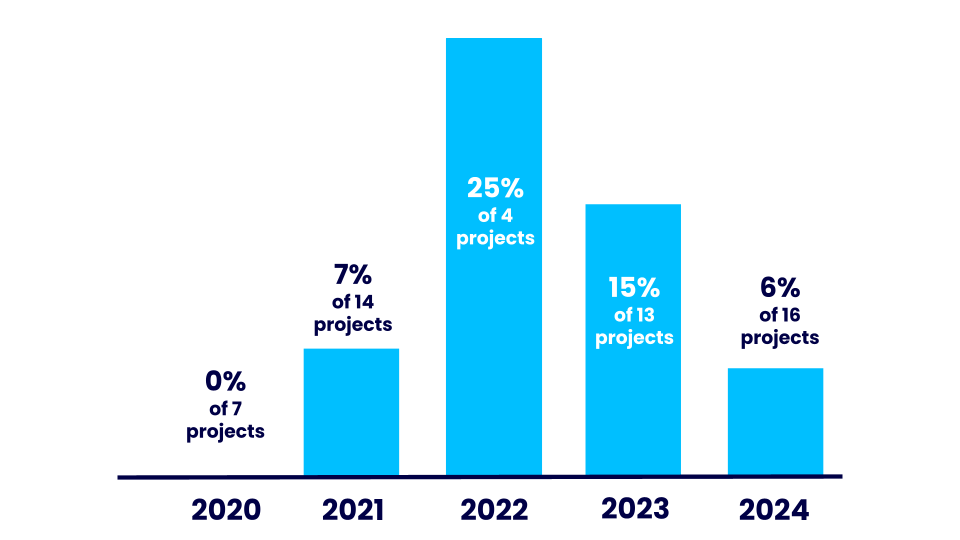
- In 2020, no new grants awarded involved animal research (0 out of 7).
- In 2021, 7% of new grants we awarded involved animal research (1 out of 14).
- In 2022, 25% of new grants we awarded involved animal research (1 out of 4).
- In 2023, 15% of new grants we awarded involved animal research (2 out of 13).
- In 2024, 6% of new grants we awarded involved animal research (1 out of 16).
The Parkinson’s Virtual Biotech is our drug discovery and development programme. It's a groundbreaking global movement to deliver life-changing new treatments in years, not decades. It has its own portfolio of projects, working in collaboration with other companies and charities, sitting separately from our research grants.
Animal research is a vital part of drug discovery projects, and all of our projects involve or have involved animals in some way.
In 2023 we had 12 active projects as part of the Parkinson’s Virtual Biotech. 6 of these involved using animal models as part of their research during that time to understand how a potential new treatment might work.
Projects that are in early drug development stages, where some of this research is still happening in the lab, are known as pre-clinical research. This will often involve animal research, after early tests in cells and tissues, to see how the new drug works, and make sure there are no harmful side effects.
At the clinical trial stage of research, animal research is no longer needed as the research group will test the new treatment to see if it works in people. However, all of the drugs that make it to this stage will have relied on animal research at some point during their development. It is a legal requirement to make sure that this happens for any new drug.
The Parkinson's Virtual Biotech is dedicated to developing new medicines to transform the lives of people with the condition.
The process of development of new drugs involves careful and rigorous testing. In the early stages of drug development this testing is mainly done in test tubes, using cells grown in a dish and increasingly using computer modelling.
Later on in development animals are required to investigate possible side effects, how the potential new drug may interact with other common medications, and how it is distributed and processed inside the body.
This testing is legally required for all new medicines.
The vast majority of experiments will be carried out using mice and rats, however regulatory guidelines require new drugs to be tested in two animal species for safety, a rodent (rat or mouse) and a non-rodent (eg. dog, pig or non-human primate), before they can be given to people in the first clinical trials.
Only once these vital tests are successfully completed, can a new drug move forward towards testing in people in clinical trials.
The UK government has developed what are often regarded as the tightest regulations for research involving animals in the world, with the highest associated standards of welfare for the animals. This legislation permits scientific procedures when the benefits that the work is likely to bring (to humans, other animals or the environment) outweigh any pain or distress that the animals may experience, and only where there are no alternatives.
All researchers who carry out studies using animals require a Home Office licence and their research facilities are regularly inspected by regulatory authorities.
Researchers are obliged to use the principles of reduction, replacement and refinement, known as the ‘3Rs’, while carrying out any research that involves animals. This means:
- reducing animal use by using the minimum number of animals required in each experiment
- replacing the use of animals with non-animal methods, or using a less sentient animal
- refining the experiments to make sure that the animal suffers as little as possible during the course of the research.
All our research grants undergo a thorough peer review by leading researchers, which tests that the ‘3Rs’ framework is being upheld. All animal studies for our Virtual Biotech projects are conducted at Home Office accredited organisations, where animal use must be reviewed and approved before they can continue.
We also encourage researchers to involve people with Parkinson’s and their loved ones in early conversations about research that involves animals. Read more about the important role of patient involvement in animal research.
We are a signatory of the Concordat on Openness, which commits us to be open and transparent about the use of animals in research. Find out more on the Concordat on Openess website.
As a member of the Association of Medical Research Charities (AMRC) we fully support its position statement on the use of animals in research. Read the position statement on the AMRC website.
Full policy statement on animals in research
This policy statement has been developed with advice and guidance from people with Parkinson's, the people who love and care for them, health and social care professionals and other experts. You can find more details in the full policy document below.
Read the full Parkinson's UK policy statement on animals in research.
Our research projects
Research we've funded has delivered groundbreaking discoveries, new treatments and better care. Find out more about the research projects we support.
Last updated December 2025. This information will be updated yearly. If you have any questions, please contact [email protected]

