Collaborating to make EJS-ACT PD happen
The world's largest Parkinson's research trial will test multiple treatments that could slow or stop Parkinson's progression.
Finding new treatments for Parkinson’s can feel like a long, slow process. But a new research study is set to revolutionise Parkinson’s research. It’s the largest UK Parkinson’s study to date and it’ll save time, money, and make the possibility of a new approved treatment for Parkinson’s a closer reality.
We’ve committed over £1.6m of funding to join this exciting collaboration of researchers, charities, and universities across the world.
The Edmond J Safra Accelerating Clinical Trials for Parkinson’s study (EJS ACT-PD) has the potential to find a treatment that can slow or stop the progression of Parkinson’s. The first drugs being trialled are in phase 3 of clinical testing. This is the final hurdle before a treatment can be approved for use.
The first two drugs to be tested in EJS ACT-PD are also already approved for use in other conditions: telmisartan is a drug currently used to lower blood pressure, and terazosin is used to treat an enlarged prostate. But there is some evidence to show they could also work to slow down Parkinson’s progression.
There’s an opportunity for 1600 people with Parkinson’s across the UK to take part from over 40 research sites, in the world’s biggest research trial for Parkinson’s. Find out more about the trial on our Take Part Hub.
We spoke to Georgia, Carroll and Jodie who are involved in planning the study.



Georgia, Research Project Manager for EJS ACT-PD shares what makes this clinical trial different.
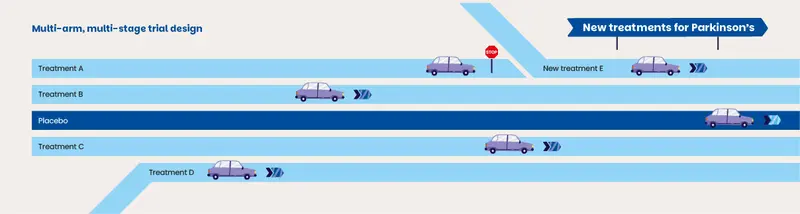
"EJS ACT-PD was set up to do things differently. In a typical clinical trial, you'll test one treatment against one placebo (dummy drug). When you finish and look at your results, you see if it’s worked, or if it hasn’t you start planning the next trial for a different treatment. That takes a lot of money, and at the end you only ever have an answer about one treatment.
"In EJS ACT-PD, we'll be using a multi-arm, multi-stage (MAMS) trial design. This means we’ll test multiple treatments at the same time, against only one placebo group."
"If a treatment doesn’t look promising, we can just stop pursuing it and add in something else. We don’t need to shut down and start again. It’s a way of getting lots of answers at once, saving money, and more importantly, time."
How did you design the study?
"We’ve worked with over 90 experts to put this study together, including scientists, healthcare professionals, health economists, charities and people with Parkinson’s and their loved ones. This means we can think about the trial from all of those different perspectives to make sure the study provides meaningful results."
You can find out more about how MAMS trials work by watching this video by Cure Parkinson's.
Carroll, diagnosed 9 years ago, and Jodie, diagnosed 8 years ago, share what it’s been like to be part of the planning of the EJS ACT-PD study.
Carroll: "Jodie and I are two of 20 people with Parkinson’s and carers that form the involvement group, and share our thoughts on how the trial should be managed, what treatment to choose, what technology should be used for monitoring, where the sites should be etc.
Jodie: "It’s our job to represent the patient voice, and this is very diverse. There are people who are newly diagnosed, and others who were diagnosed 20 years ago. We all have different priorities. That means sometimes we disagree over what is a reasonable side effect, or a practical time commitment. But we have a lot of respect for each other and the leadership team really values all of our views."
What are you most looking forward to as EJS ACT-PD gets underway?
Carroll: "I think the trial has potential to find a major breakthrough. We know that Parkinson’s gets worse over time, and there are currently no treatments to slow or stop it. EJS ACT-PD is going to prioritise treatments that are aiming to do just that. And by testing the treatments in a quicker, more efficient way, hopefully we can get to one faster.
"I’m looking forward to seeing the ideas we’ve worked on over the last 5 years in action!"
Jodie: "Ultimately what I’m most interested in is getting a new treatment approved for Parkinson’s. Studies like EJS ACT-PD are tipping the odds of that happening in our favour. It’s been a huge effort so far and more to come, but we must never give up."
Interested in taking part?
The EJS ACT-PD trial will recruit over 1,600 people with Parkinson's and their loved ones, across 40 sites in the UK. Find out more about how you can get involved on our Take Part Hub.
This article featured in the Summer 2025 edition of Progress, our research magazine. Read the full edition on our website.

